Benefits of Chlorhexidine in the Adhesion Process
By Jeffrey Bonk on June 15, 2023 | commentsAdhesion to tooth structure is an integral part of today’s dentistry. Esthetics, function, longevity—these are all related to the ultimate success of bonded restorative procedures. Direct and indirect restorations are dependent upon the adhesive protocols used. Restoration durability is the key to long-term success and predictable outcomes. In this article, we will look at the role and benefits of chlorhexidine in helping you achieve those successful outcomes.
Adhesion to tooth structure is predictable. Enamel and dentin are two distinctly different structures. Because enamel is approximately 95% inorganic hydroxyapatite crystals, etching enamel with phosphoric acid creates significant micropores into which resin may infiltrate. Enamel bonding is approximately 60 MPa in strength and creates a definitive seal to resist microleakage and secondary decay. John Burgess, a well-known researcher from LSU, is quoted as saying “the seal is the deal” when referencing enamel adhesion. Nothing is better than the adhesive bond to enamel.
Dentin, on the other hand, is a totally different structure. It is composed of dense collagen fibrils wrapped in hydroxyapatite, with water interposed throughout the matrix. The high humidity (~ 20% water) and limited inorganic content (approx. 40-50% hydroxyapatite crystals) significantly affects the adhesive strength and longevity of the bonded components. Bonding to dentin is predictable, but much less strong (approximately 30 MPa) than the bond to enamel.
The adhesive's goal is to create a long-lasting and durable connection of resin to these dental hard structures.

The Role and Benefits of Chlorhexidine in the Bonding Process
The strategies for adhering resin to tooth structure are either etch-and-rinse or self-etching protocols. The sequences and processes for each are definitive and well-documented and should be followed precisely for effective adhesion outcomes.
Whatever strategy you use, the ultimate objective is longevity and predictability of the hybrid layer. To gain the expected duration of adhesive serviceability, exacting technique and appropriate actions must be implemented and integrated to avoid early degradation of the resin matrix. Residual water remaining in the deepest portions of the dentin matrix, along with activation of matrix metalloproteinases (MMPs) can result in hydrolysis of the organic collagen component and disruption of the hybrid layer seal. Specific degradation inhibitors, specifically chlorhexidine, can interrupt this destructive process and help maintain the integrity of the hybrid layer. It is important for dentists to be aware of this degenerative process and to understand the tools and techniques to limit hybrid layer destruction.
MMPs are collagenic enzymes contained within the dentin structure that manage the stability and equilibrium of the organic matrix structure. MMPs can be found not only in dentin but also in bone and soft tissues (e.g., periodontium). These proteases remain quiescent until activated by irritants such as the hydrolytic effects from water sorption, an acidic environment, or retained and/or un-evaporated resin monomers. This activation results in the biodegradation of the dentin adhesive interfaces. To achieve long-term bond stability, it is imperative to prevent or inhibit this process.
Chlorhexidine digluconate (CHX) is the most well-documented inhibitor of this degradative process. Although the exact mechanism of how CHX interferes with MMP activation is unclear, it has been shown to reduce the destructive effects within the dentin matrix. CHX binds with zinc and calcium ions to reduce MMP activation.
CHX can be incorporated into the resin bonding process in three ways:
- It can be integrated into the etching agent, which is then rinsed away from the surface.
- It can be incorporated directly into the resin adhesive.
- It can be applied as a separate solution (2% CHX) directly on the dentin following etching, then rinsed away. This is the most common approach.
Consepsis© (Ultradent) is the most known dental application material for this purpose. Gostemeyer et.al. has performed research that shows that dentists incorporating this CHX inhibition protocol will not harm or diminish the bonding process and hybrid layer formation. Breschi has recently discovered that the CHX ion remains present and active within the resin layer for 10 years. The MMP inhibiting effect is an important function.
How Chlorhexidine Works Within the Hybrid Layer
Application of CHX has a positive impactful effect on the longevity and durability of the adhesive layer. Having a “visual “perspective of the mechanism by which CHX works within the hybrid layer will provide greater understanding and motivation toward implementing this step into the bonding process.

The collagen fibrils within the dentin matrix are coated with hydroxyapatite crystals. These inorganic crystals provide rigidity to the collagen fibrils. Interposed and intermixed among these fibrils are the matrix metalloproteinases (MMPs), whose purpose is to regulate the dentin structure and maintain its integrity.

Etching of the enamel and dentin are critical steps in the adhesive process to gain marginal seal and create a strong resin connection to tooth structure. Etching enamel provides for the micro-mechanical locking of resin to this inorganic structure. Etching of both structures removes hydroxyapatite crystals. When using the etch-and-rinse strategy, the hydroxy appetite crystals of the dentin are removed along with the smear layer, to a depth of 5-8 microns. Following rinsing of the etchant, the collagen matrix becomes an entangled web of intertwined fibrils. With most of the hydroxyapatite removed, only the denuded fibrils with MMP proteinase remain. Residual water can remain trapped within the deepest portions of the etched dentin, which can interfere with hybrid layer integrity (which will be discussed later).
When using self-etch strategies, the self-etching monomers are applied but not rinsed. Rather, the smear layer becomes incorporated into the resin layer. With this technique, there is a reduced chance of MMP activity from denuded or exposed collagen fibrils. However, hydrolytic degradation may still occur depending upon the type of monomers applied. CHX can have positive impacts on self-etching strategies as well.

A separate solution of chlorhexidine is applied to the denuded collagen fibrils in multiple applications with agitation. Using 0.2% chlorhexidine will allow for the CHX ion to attach directly to the collagen. This ionic connection is strong and resilient. The chlorhexidine is applied for 15 seconds and rinsed away. CHX exerts its inhibitory effects within the attached and surrounding collagen fibrils. Diagrammatically, it can be visualized as a “circle of protection” for the hydroxyapatite-denuded collagen fibrils. Because of the proximity of the fibrils, an “overlapping protecting mechanism” helps to maintain the integrity of the subsequent hybrid layer.”
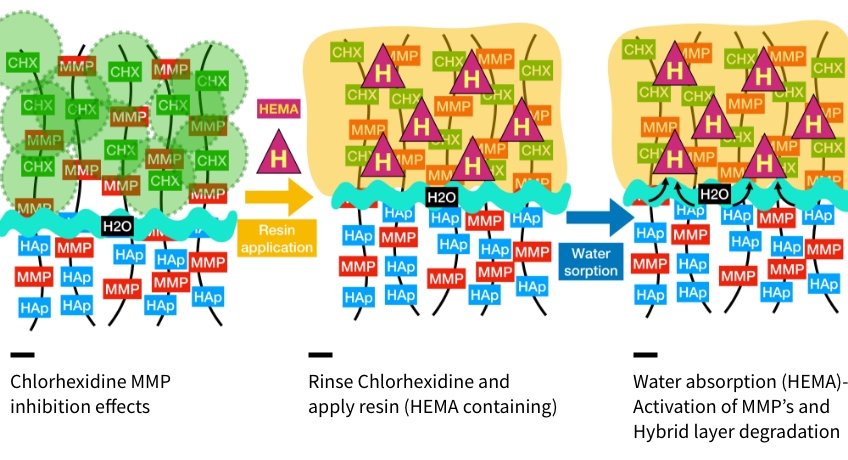
Resin monomers are then applied to the exposed dentin matrix. The hydrophilic monomers aid in supplanting and exchanging the water within the matrix to “prime” the collagen for hydrophobic resin attachment. Again, multiple applications with agitation will help with removing excess water and prime the dentin. The resin monomers are air-dried.

Resin monomers contain many ingredients to help adhere these products to collagen. One of the most common ingredients is hydroxyethyl methacrylate (HEMA). This monomer is very hydrophilic, so it effectively chases water and primes the collagen for the hydrophobic resin adhesive. This monomer is used in most resin adhesives, especially in the newer universal or “multi-mode” adhesives. One particularly important detrimental side effect of HEMA is that it readily absorbs water. Because of this property, HEMA may contribute to hybrid layer destruction and MMP activation.

The action of HEMA absorbing water creates “voids” in the hybrid layer. The loss of resin matrix integrity results in exposure of collagen fibrils. Exposed collagen undergoes hydrolytic degradation very easily. Loss of the fibril structure results in leakage and breakdown of the resin/tooth matrix. The benefit of chlorhexidine ions helps to reduce this effect and support the integrity of the hybrid layer matrix. Without the presence of chlorhexidine, the action of HEMA will continue and create “water trees” that permeate the hybrid layer and lead to integrity failure. Researchers Pashley and Tay are credited with identifying the hydrolysis of collagen associated with water absorption through the hybrid layer.
Managing the longevity and predictability of dental adhesion is paramount for success in dentistry today. Using appropriate protocols to ensure bond strength longevity is a simple and efficient process that has longstanding positive effects. The benefits of chlorhexidine as a tool to prevent early bond degradation are highly effective. I hope this article provides insights that help you integrate this material—and this strategy—into your bonding protocols.
Jeffrey Bonk, D.D.S., is a member of Spear Resident Faculty.
References
Meerbeek, B. V., Yoshihara, K., Van Landuyt, K., Yoshida, Y., & Peumans, M. (2020). From Buonocore's Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. Journal of Adhesive Dentistry, 22(1).
Gostemeyer, G., & Schwendicke, F. (2016). Inhibition of hybrid layer degradation by cavity pretreatment: meta-and trial sequential analysis. Journal of dentistry, 49, 14-21.
Pashley, D. H., Tay, F. R., Yiu, C. K. Y., Hashimoto, M., Breschi, L., Carvalho, R. M. D., & Ito, S. (2004). Collagen degradation by host-derived enzymes during aging. Journal of dental research, 83(3), 216-221.
Betancourt, D. E., Baldion, P. A., & Castellanos, J. E. (2019). Resin-dentin bonding interface: Mechanisms of degradation and strategies for stabilization of the hybrid layer. International journal of biomaterials, 2019.
Zhou, J., Tan, J., Chen, L., Li, D., & Tan, Y. (2009). The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. Journal of dentistry, 37(10), 807-812.